

Additive Engineering with Triple Cations and Bifunctional Sulfamic Acid for Tin Perovskite Solar Cells
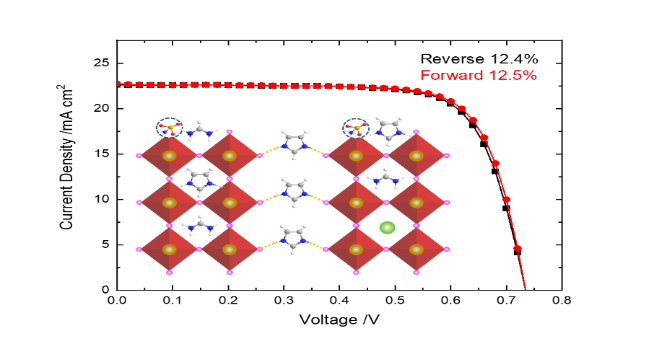
Imidazolium (IM) and cesium (Cs) were treated as A-site cationic additives for triiodide tin perovskite solar cell (FASnI3, FA: formamidinium) with sulfamic acid (SA) as a bifunctional additive in varied proportions. IM has an ionic radius (258 pm) similar to that of FA (253 pm) but with a tight aromatic structure which is feasible to passivate the crystal defects and help for perovskite crystallization confirmed by the XRD and SEM measurements. Cs has a smaller size (167 pm) but small amounts of Cs in tin perovskite crystal can stabilize the crystal structure and to decrease trap state densities confirmed by the TCSPC lifetime measurements. The existence of both IM and Cs in the triple cationic perovskite significantly enhance the Sn2+/Sn4+ ratios confirmed by the XPS measurements. TOF-SIMS measurements show the passivation effect of both IM and SA occurring on the surface of the film. The bifunctional SA additive can occupy the iodine vacancies in tin perovskites to passivate the uncoordinated Sn atoms and to passivate the surface defects effectively. Furthermore, SA has the effect to reduce Sn4+ back to Sn2+ via its ammonia/acid form converted from its zwitterionic form upon irradiation observed from in-situ XPS measurements. The hybrid device was optimized to attain PCE 12.5 % at the perovskite structure Cs0.02IM0.1FA0.88SnI3+1% SA with superior enduring stability.
Chun-Hsiao Kuan, Juin-Min Chih, Yu-Cheng Chen, Bo-Hong Liu, Chia-Hsin Wang, Cheng-Hung Hou, Jing-Jong Shyue, and Eric Wei-Guang Diau,* “Additive Engineering with Triple Cations and Bifunctional Sulfamic Acid for Tin Perovskite Solar Cells Attaining PCE 12.5 % without Hysteresis”, ACS Energy Lett., 7, 4436−4442 (2022).
Self-Photocatalytic Splitting of Carbon Dioxide using Co-Cationic PeNC in Absence of Water
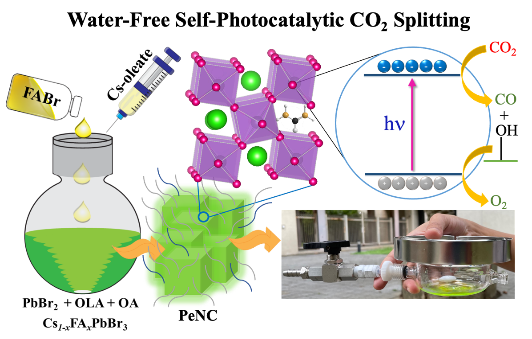
Environmental concerns demand efficient removal of CO2, a major greenhouse gas. For this purpose, a traditional chemical strategy implements a catalytic reaction to reduce CO2 to CO, with H2O as sacrificial agent in a counter reaction to generate O2. Herein we report self-photocatalytic CO2 splitting to generate CO and O2 in absence of H2O, using perovskite nanocrystal Cs0.55FA0.45PbBr3 (CF). We obtained a record production rate 105 μmol g-1h-1 of CO, which is three times than that with CsPbBr3 (CS) as photocatalyst at the gas-solid interface. During photocatalytic reaction, a transition from orthorhombic to cubic phase occurred with an enlarged crystal size through the effect of Ostwald ripening, for which the CO yield approached 3.1 mmol g-1 within reaction period ~60 h. During this Ostwald ripening process, both FA and oleylammonium cations were released to provide the proton source for the CO2 reduction to proceed and generate hydroxyl species required for oxidation. A self-photocatalysis mechanism involving bound hydroxyls is proposed and confirmed by the yield of O2 being half that of CO and the isotopic experiment using C18O2 as a photoreactant.
Sumit S. Bhosale, Aparna K. Kharade, Sudhakar Narra, Sue-min Chang* and Eric Wei-Guang Diau*, “Self-Photocatalytic Splitting of Carbon Dioxide using Co-cationic Perovskite Nanocrystals in the Absence of Water”,ACS Energy Lett., 8, 280-288 (2023).